shelf life calculator for pharmaceutical products
Therefore accurately determining the price of Q10 is very important. Also it is necessary to find the mark.

Shelf Life Calculator For Composites And Other Materials
A pharmaceutical product is typically manufactured in batches.

. These concepts are already in use but not named as such. Is stored under the conditions defined on the container label 2 PDU4 Team Novartis East Hanover New Jersey USA. Shelf life calculator for pharmaceutical products Saturday June 4 2022 Edit.
Guidelines on Stability Studies of Pharmaceutical Products and Shelf Life Estimation. A batch is a fixed quantity of product for example 100000 tablets. Expiry with date in days in months or in years.
At that time Q10n 3 25 156. Westpak does not recommend aging packaging materials at temperatures exceeding 60ºC. Shelf Life Calculation Of Drugs.
The five new terms that are necessary for a coherent discussion of shelf life are. Although this is an accepted definition a crucial-for-imple- 3. This online service helps you to know how long your product is in good condition.
In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field. The shelf-life of a drug product is the time that the average drug charac- teristic eg potency remains within an approved specification after manufacture. Nonclinical and Pharmaceutical Sciences Statistics Merck West within the approved shelf life specification provided that it Point Pennsylvania USA.
The article presents some statistical evaluation of stability data in order to determine the shelf-life of pharmaceutical products and. What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. Abstract and Figures.
Accelerated Aging Time AAT Desired Real Time RT Q10 TAA TRT 10 NOTES The calculated AAT is typically rounded up to the nearest whole day. Common TAAs are 50ºC 55ºC and 60ºC. Each batch is distinguished by its own shelf life which can be called the true batch shelf life.
The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date. Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services. Accelerated stability testing and shelf-life calculation.
For example A manufacturing date B expiry date C Date at 75 remaining. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration date of a drug product and the stability testing needed to ensure that. Arrhenius plot to get k at 25 ºC and from this we can calculate the shelf-.
Expected shelf life is usually. Statistica Sinica 112001 737-745 DRUG SHELF-LIFE ESTIMATION Jun Shao and Shein-Chung Chow University of Wisconsin and StatPlus Inc. True shelf life estimated shelf life supported shelf life maximum shelf life and labeled shelf life.
32 days x 156 500 days. The higher the Q10 the longer the expiry date. This document assists with establishing the expiration period of a production bath of a medicinal productIt is not applicable to biological medicinal products such as vaccines sera toxins and allergens products derived from human blood and plasma as well as medicinal products prepared biotechnologically.
This guideline applies to human and veterinary medicines. Shelf life Pharmaceutical Stability Shelf Life August 1 2010 2 Definitions of Shelf Life ICH Q1E ICH Guideline Q1E defines shelf life as The shelf life of a pharmaceutical product is the maximum time at which the true mean response of a stability limiting characteristic crosses th e acceptance criterion. Ad Browse Discover Thousands of Medicine Book Titles for Less.
Input the TAA and TRT values both in Celsius for your product. The quality within each step influences the quality of the resulting knowledge. Any drug product intended for reconstitution and not bearing an expiration date for the unreconstituted product and another expiration date for the product after reconstitution is considered to be.
This guideline applies to human and veterinary medicines. Ambient temperature is typically between 20ºC to 25ºC. The batch is a single sample of the pharmaceutical products manufacturing process at.
As the example above if Q10 2 the normal shelf life at 25 C will be 181 days 27 times less than in case Q10 3. Establishing the Shelf Life of Pharmaceutical Products. Calculate half-life and shelf life of pharmaceutical products and drugs.
Which equation is used for predicting the shelf life of a drug product. How do you calculate remaining shelf life. Batch and Product Shelf Life.
The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life. Then you may use the formula 025B2075A2 in C2 on my sample please remember to set the cells with results as Date. Establishing the Shelf Life of Pharmaceutical Products QUANTILE REGRESSION CALIBRATION At least three distinct steps are necessary to gain knowledge and understanding of a product or process.

Shelf Life Calculator For Composites And Other Materials
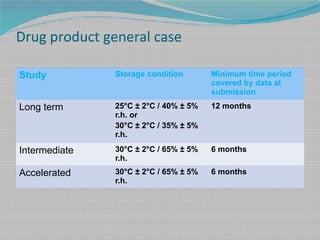
Stability Testing And Shelf Life Estimation

Shelf Life Of Foods First Order Kinetics Example Youtube

Microsoft Excel Shelf Life Calculate On Which Day Left Equals 75 Super User

Shelf Life Calculator For Composites And Other Materials
Stability Testing And Shelf Life Estimation

Specialty Pharmacy The Complete Pharmacist Specialty Pharmacy Pharmacy National Cancer Institute

Shelf Life Calculator For Composites And Other Materials
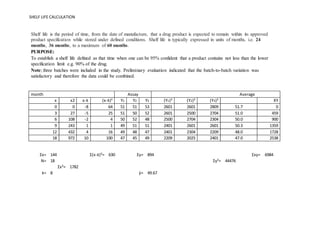
Shelf Life Calculation Of Drugs

Shelf Life Calculation Of Drugs

Analyzing Data From Stability Studies Youtube

Checking And Calculating The Shelf Life Expiration Date Sap Help Portal

Free Legacy Food Endure Probiotic By Legacy Premium 49 99 Http Www Freelegacyfood Com Endure Probiotic B Probiotics Freeze Drying Food Digestive Health

Shelf Life Calculator For Composites And Other Materials

Fda Approves Drug To Treat Dangerously Low Blood Pressure

Shelf Life Calculator For Composites And Other Materials
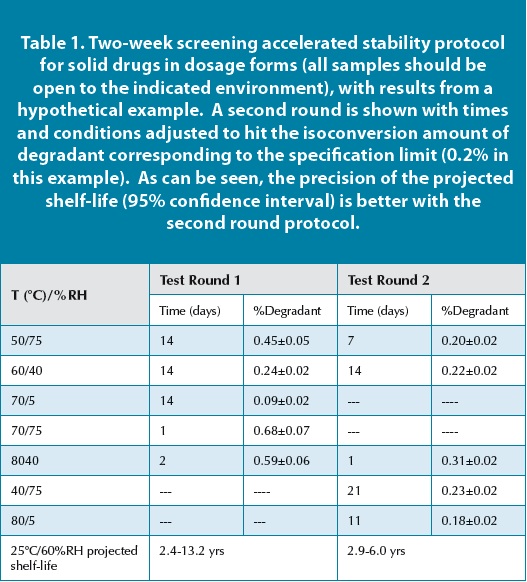
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services